Products
Effects and safety of NKCP
Several types of thrombolytic enzymes are known to be produced by Bacillus subtilis var. natto. One of them, bacillopeptidase F, is the main component of NKCP. Bacillopeptidase F has been confirmed to have three functions: (1) anticoagulant, (2) blood viscosity reduction, and (3) thrombolytic actions. In vitro and clinical studies have demonstrated that the consistent intake of NKCP over a prolonged period helps to maintain normal blood circulation. In addition, NKCP is made from natto (fermented soybeans), which has been consumed in Japan for a long time. We have placed great importance on safety of our ingredients from the earliest stages of product development. The safety of NKCP has been demonstrated in animal and human safety studies.
Function of NKCP
The human body contains blood vessels that, including capillaries, that span approximately 100,000 km, equivalent to two and a half times the Earth’s circumference. Blood leaving the heart flows through blood vessels to deliver oxygen and nutrients to every cell of the body and to collect waste products.

However, obesity, lack of exercise, and cholesterol-rich diets in modern society can lead to narrowing of blood vessels due to arteriosclerosis, which worsens blood flow and thereby makes it easier for blood clots to form.
NKCP, which contains bacillopeptidase F, helps prevent thrombosis (e.g., cerebral and myocardial infarctions) through the triple function of (1) anticoagulation, (2) blood viscosity reduction, and (3) thrombolytic action.
Mechanism of Action
The coagulation/fibrinolysis system of blood is composed of complex reactions caused by the interaction of numerous factors. This mechanism ensures that activation of a single factor does not disrupt the whole balance. However, if the balance is disrupted for some reason, blood clots can easily form or become difficult to dissolve. Moreover, restoring the balance once it is disturbed is not easy.
NKCP suppresses the formation of blood clots and the increase of blood viscosity, thereby helping to maintain proper balance and blood flow to all parts of the body.

Scientific Research
① Anticoagulant effect
② Action of preventing increase in blood viscosity
③ Thrombolytic effect
④ Clinical Study Results
The three functions of NKCP are demonstrated to improve a variety of symptoms in clinical trials.
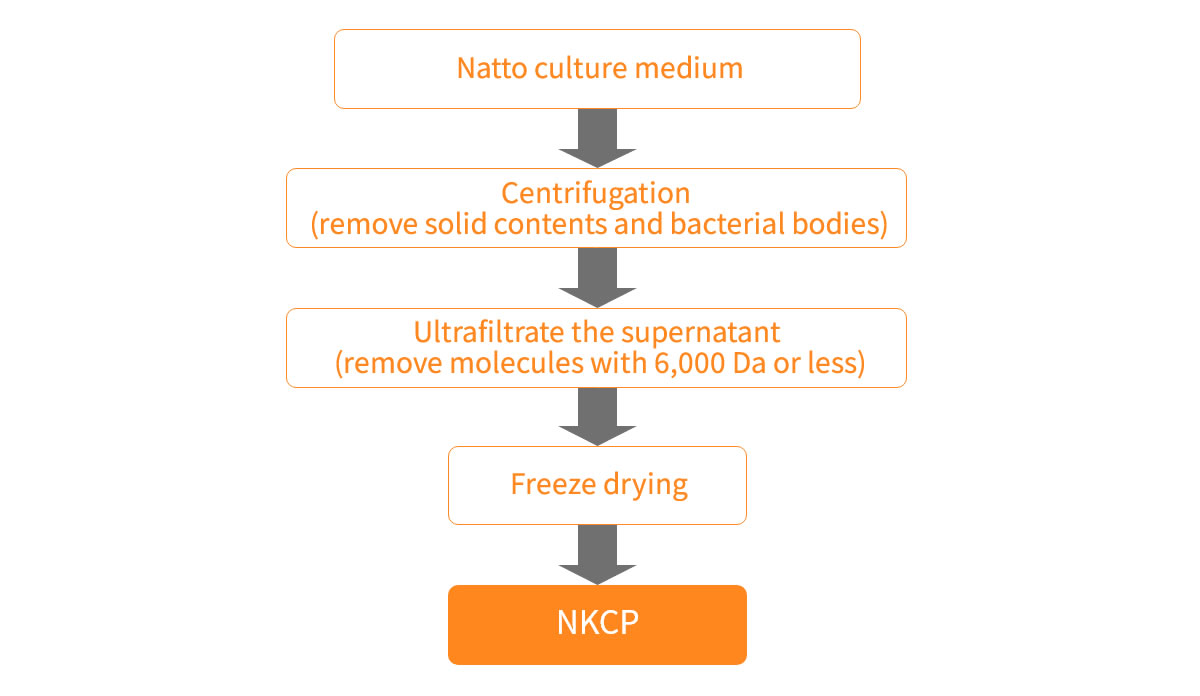
Manufacturing Process
Bacillus natto is cultured in liquid under conditions that efficiently produce bacillopeptidase F. From this culture, bacterial bodies and unwanted substances are removed, and the culture is lyophilized into powder. NKCP is a mixed powder prepared so that the enzyme activity and protein content remain constant.
Safety Data
Suggested daily dose
Based on the results of animal and human studies, we recommend to taking 125 mg to 500 mg of NKCP per day.
Safety
| Acute toxicity (Rat) | LD50>5,000 mg/kg |
|---|---|
| Repeated dose toxicity (rat) | NOAEL (90 days) Male > 1,325 mg/kg/day Female > 1,541 mg/kg/day |
| Mutagenicity | Negative (± metabolic activation) |
| Antigenicity (guinea pigs) |
Negative for active systemic anaphylactic reaction (ASA) and passive cutaneous anaphylactic reaction (PCA) |
| Effect on bleeding time (rats) | In rats orally given NKCP, a 0.5mm incision was made in the tail tip after 1 hour to measure bleeding time. NKCP at 300 mg/kg did not prolong the bleeding time. |
| Interaction with warfarin (rats) | NKCP at 250 mg/kg was administered into the duodenum by the in situ loop method in rats, in which bleeding time was delayed by treatment with warfarin, and blood collected after 6 hours was measured for coagulation time. The warfarin treatment significantly prolonged the coagulation time in comparison with the control group, but no added delay of coagulation was observed in the warfarin + NKCP treatment group compared with warfarin treatment group. |
| Long-term administration (humans) |
Twenty-three healthy adults were given NKCP at 250 mg/day for 12 weeks, and no clinically significant adverse events were observed. There were no statistically significant changes in hematological or biochemistry tests. Five healthy adults were given NKCP at 750 mg/day for 6 consecutive weeks to study and observe changes in laboratory test values (hematological tests, biochemistry tests, and blood coagulation/fibrinolysis parameters) and adverse events. As a result, ELT shortened, t-PA decreased, and thromboplastinogen activity test (TAT) increased but all values were within normal range. In addition, no adverse events were observed, suggesting NKCP safety. |
| High dose administration (humans) | Eight healthy adults were given NKCP at 1,250 mg/day for 7 consecutive days. Observation of clinical signs and laboratory tests were utilized to evaluate NKCP safety. There were no clinically significant adverse events. There were no abnormal changes in hematological or biochemistry tests. |
Assays
| 1. Peptidase Activity (synthetic substrate method) |
|---|
| The sample solution is incubated at 37°C with the synthetic chromogenic substrate S-2251 (H-D-valyl-L-leucyl-L-lysine-p-nitroanilide dihydrochloride) as a substrate and the absorbance at 405nm is determined. The enzyme activity is defined as 1 unit when 1nmol of p-nitroaniline per minute is released. |
| 2. Bacillus subtilis var. natto-produced Protein |
| ELISA (enzyme-linked immunosorbent assay) uses rabbit-specific antibodies to the Bacillus subtilis var. natto-produced protein, responsible for the peptidase activity, to measure the amount of antigen reacting with the specific antibodies. |
